Diamonds are among the most admired and studied natural materials in the world. Known for their extraordinary brilliance and unmatched hardness, diamonds serve both aesthetic and industrial purposes. This guide offers a comprehensive look at the physical and chemical properties of diamonds, their natural and synthetic origins, and the various ways they are used across industries and cultures.
A Complete Guide to Diamond Properties and Uses

What Is a Diamond?
A diamond is a crystalline form of carbon arranged in a cubic (isometric) structure. Under extreme pressure and temperature conditions deep within the Earth’s mantle, carbon atoms bond in a specific way that gives diamonds their incredible hardness and optical properties. This unique atomic structure results in a gemstone prized not only for its beauty but also for its utility.
Diamonds can be found in a variety of colors—from colorless to shades of yellow, brown, blue, green, and even pink or red—depending on the presence of trace elements and structural anomalies. While colorless diamonds are the most highly valued in the jewelry industry, fancy-colored diamonds have carved out their own niche among collectors and connoisseurs.
Physical and Chemical Properties

1. Hardness
- Diamonds are the hardest known natural material, rated 10 on the Mohs scale.
- Their hardness makes them resistant to scratching by any other substance, but they can still be fractured by sharp impacts due to their crystal structure.
2. Optical Properties
- Diamonds are highly refractive (with a refractive index of 2.42), which allows them to bend light dramatically.
- Their dispersion (ability to separate light into different colors) contributes to the colorful “fire” or sparkle that makes diamonds so visually captivating.
3. Thermal Conductivity
- Diamonds conduct heat better than most materials, making them extremely useful in high-performance electronics.
- This property also helps in industrial applications like heat sinks and semiconductor components.
4. Chemical Stability
- Composed entirely of carbon, diamonds are chemically inert to most acids and bases.
- They can burn at very high temperatures in an oxygen-rich environment, converting to carbon dioxide.
5. Crystal Structure
- The cubic crystal system gives diamonds their geometric strength.
- Each carbon atom is bonded to four others in a tetrahedral shape, which contributes to the overall symmetry and robustness of the diamond.
Natural vs. Synthetic Diamonds

Natural Diamonds
- Formed over billions of years, natural diamonds are created under high-pressure, high-temperature conditions deep within the Earth.
- They are typically brought to the surface via volcanic activity through kimberlite pipes or lamproite pipes.
- Their age and natural origin contribute to their rarity and value, particularly in gem-quality stones.
Synthetic Diamonds
- Created in controlled environments using methods such as:
- High Pressure High Temperature (HPHT): Mimics natural diamond-forming conditions.
- Chemical Vapor Deposition (CVD): Involves growing diamonds layer by layer from carbon-rich gases.
- These diamonds are virtually indistinguishable from natural ones in chemical and physical properties but can often be identified through specialized lab analysis.
- Lab-grown diamonds are generally more affordable and offer a more sustainable and ethical alternative.
Uses of Diamonds

1. Jewelry
- Diamonds are the most popular gemstones for engagement rings, wedding bands, and high-end fashion accessories.
- Their brilliance and ability to withstand daily wear make them ideal for heirloom and everyday jewelry.
- Settings range from classic solitaires to intricate pavé and halo designs, and diamonds are also used in watches and high jewelry collections.
2. Industrial Applications
- Diamonds are used in cutting, grinding, drilling, and polishing tools due to their extreme hardness.
- Common applications include:
- Diamond-tipped drill bits in mining and construction
- Abrasive pastes for precision polishing
- Cutting wheels for manufacturing semiconductors and ceramics
3. Electronics and Optics
- Their exceptional thermal conductivity and electrical insulating properties make diamonds ideal for high-performance electronics.
- Used in:
- Laser optics
- Heat spreaders in microelectronics
- Semiconductor devices for high-voltage or high-frequency applications
- Experimental applications in quantum computing and photonics
4. Medical Equipment
- Diamonds are used in medical tools that require exceptional sharpness and durability:
- Scalpels and blades for eye surgery (such as LASIK procedures)
- Dental instruments with diamond coatings for precision and longevity
- Implants and diagnostic devices due to diamond’s biocompatibility and non-reactive nature
5. Scientific Research
- High-purity synthetic diamonds are essential in scientific experiments:
- Diamond anvil cells recreate extreme pressure conditions to study Earth’s interior and new material phases.
- Used in spectrometers, particle detectors, and other precision instruments requiring stable, inert materials.
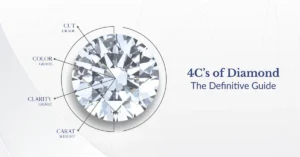
The 4Cs of Diamonds (Jewelry Grade)
When buying or evaluating a diamond, four key criteria—known as the 4Cs—are used:
- Carat – Refers to the weight of the diamond. One carat is equal to 200 milligrams. Larger diamonds are exponentially rarer and more expensive.
- Cut – Influences how well the diamond reflects light. Cuts like brilliant, princess, cushion, or emerald can enhance sparkle and optical performance.
- Color – Rated from D (colorless) to Z (light yellow or brown). The less color, the more desirable the diamond.
- Clarity – Measures the presence of inclusions (internal flaws) or blemishes (surface flaws). Ranges from Flawless (FL) to Included (I1, I2, I3).
Each of these factors influences the value, appearance, and durability of a diamond.
Fancy-Colored Diamonds
While white diamonds dominate the market, fancy-colored diamonds have gained popularity for their uniqueness. Natural hues like blue, pink, green, and red are extremely rare and valuable. Some colored diamonds owe their color to the presence of trace elements (boron for blue, nitrogen for yellow), while others gain color through structural distortions.
Ethical and Environmental Considerations
With increasing awareness around sustainability and social responsibility, ethical sourcing has become a key factor in diamond purchasing:
- Conflict-free diamonds: Ensuring that diamonds are mined in conditions that do not fund violence or human rights abuses. The Kimberley Process is one regulatory effort to prevent the trade of conflict diamonds.
- Lab-grown alternatives: Offer consumers environmentally sustainable and ethically sound options.
- Recycled diamonds: Gaining traction among eco-conscious buyers, these are repurposed stones with minimal environmental impact.
Diamond Imitations vs. Authentic Diamonds
Many materials resemble diamonds but differ in properties:
- Cubic Zirconia (CZ): A common simulant with lower hardness and brilliance
- Moissanite: Very similar in appearance but has a higher dispersion (more fire)
- White sapphire and quartz: Less brilliance, lower durability
Professional gemologists use tools such as refractometers, UV lights, and thermal conductivity testers to distinguish real diamonds from imitations.
Investing in Diamonds
Though traditionally associated with jewelry, diamonds are increasingly viewed as an investment commodity:
- Loose diamonds, especially those with exceptional grades, are sometimes used as a hedge against inflation
- Colored diamonds and rare cuts can appreciate in value over time
- Investment-grade diamonds should come with certification from reputable labs such as GIA or IGI
Diamonds are more than just symbols of luxury and love. Their unique combination of optical brilliance, extreme hardness, and thermal properties makes them invaluable across diverse industries. From adorning engagement rings to advancing cutting-edge technology, diamonds represent both natural wonder and human innovation.
As both a fashion statement and a functional material, the diamond’s appeal continues to evolve. Whether you’re selecting a gem for its sparkle, exploring its industrial benefits, or considering its ethical footprint, diamonds remain one of nature’s most extraordinary creations.